Squamous Cell Carcinoma: The Definitive Guide
This is the definitive guide on squamous cell carcinoma. This guide is more in-depth than your typical information on SCC. We address questions that often go unanswered. We also cover interesting facts, this includes:
- Why doctors choose a particular treatment instead of others – we discuss the pros and cons backed up with real-world, evidence
- Are men or women at higher risk for SCC?
- Which common blood pressure medications increase your risk of developing one?
- Why NZ does not record statistics for SCC?
- Why does removal surgery for it result in such a large scar?
- How much does alcohol increase your risk for SCC?
- Which cheap, cream-based treatments can help prevent the need for surgery later on?
Squamous cells produce keratin which is the main protein component that makes up skin, hair and nails. SCCs are thought to make up about 8% of all cancers worldwide making them the second most cancer worldwide. It is classified as a non-melanoma (keratinocyte) skin cancer (NMSC) making up 20% of NMSCs.
SCCs can invade tissues and cause significant destruction to the local area if left untreated. This includes invasion into muscle, bone, and nerves. About 5% of SCCs spread to distant sites of the body (metastasise).
Most SCCs are curable and typically treated by surgical removal (excision). However, less invasive treatment options are possible in appropriate situations – particularly if the squamous cell carcinoma is early or superficial.
How common are SCCs (epidemiology)?
It is difficult to know precisely how common SCCs are as governments generally don’t record statistics for SCCs. As an example, the New Zealand Cancer Registry (NZCR) records all cancers but specifically excludes cutaneous SCC – except when they arise in the genitalia. In 1958, the registry decided to stop registering SCCs due to resource constraints – there were just too many SCCs! However, various estimates suggest at least 30,000 SCCs will be diagnosed each year in New Zealand (about 0.7% of the adult population).
Different countries have varying rates of skin cancer. It is most common in New Zealand and Australia, which have rates approximately 10 times higher than in Northern European countries, and about double the risk in the USA. In Australia, there are approximately 130,000 SCCs diagnosed per year. This compares with a rate of about 700,000 SCCs per year in the United States and about a 30% lifetime risk for white Americans.
Within Australia, Northern regions (closer to the equator) have rates of squamous cell carcinoma that are triple the rate of Southern regions. SCCs are approximately twice as common amongst men, compared to women. Presumably, this is due to better sun protection behaviours by women.
Who gets SCCs (risk factors)?
Sun exposure is by far the most important risk factor for developing SCCs. Ultraviolet radiation causes damage to the DNA in the skin and over time this can progress to developing SCC.
Most other individual risk factors are a function of sun exposure including lifestyle, occupational sun exposure, fair skin, and older age.
- Occupational sun exposure is considered a major risk factor and doubles the risk for SCC. Non-occupational sun exposure is not thought to be a risk factor for developing one.
- Chronic and cumulative sun exposure is the most important pattern of risk for SCC. This contrasts with the intense and intermittent exposure pattern that is more important for BCCs and melanoma.
- Fair complexion is a risk factor for developing skin cancer. Individuals with fair skin develop SCC at 100 times the rate of those with pigmented skin.
- A family history of SCC confers a 2-3 times risk for developing SCC
- One of the biggest risk factors for developing an SCC is having previously had one.
- About 42% of people will develop another SCC within five years of an SCC diagnosis.
- For those with a history of at least two SCCs, their risk is 72% for developing another SCC within 5 years.
- Tanning bed use increases the risk of developing SCCs at an earlier age. Studies have shown that tanning bed users have a 67% higher risk of developing an SCC.
- Some medications may increase the risk of SCCs by increasing the skin’s sensitivity to light. These include common blood pressure medications/diuretics. Medications that have been found to increase the risk are listed in the following table:
| Medication | Risk Increase (%) |
| Thiazide diuretics (e.g., hydrochlorthiazide) | 93% |
| Statins (simvastatin, atorvastatin, pravastatin etc.) | 0-14% (conflicting studies) |
| Voriconazole | 70% |
| Azathioprine | 56% |
Radiation exposure such as from cancer treatment, or occupational exposure may also increase the risk. The risk is thought to be 3-5 times higher for those who have been exposed to radiation. Interestingly, the atomic bombings of Japan are not thought to have increased the risk of SCC, whereas it does increase the risk of basal cell carcinomas (BCC). It is thought that the basal cells involved in BCC are more susceptible to radiation.
Genetic disorders
There are a few genetic disorders that substantially increase the risk of developing SCCs. While these are rare, they have helped our understanding of the pathophysiology of SCCs and highlighted potential therapeutic targets.
- Xeroderma pigmentosum individuals develop SCCs at about nine years of age and their risk is about 2,000 times the general population. The condition is autosomal recessive and impairs DNA repair due to UV damage. They also suffer from sensitivity to light, premature ageing and mottled pigmentation.
- Epidermolysis bullosa is a rare, inherited condition that affects cell adhesion (the ability of cells to hold together) and is characterized by blistering lesions. They have an increased risk of developing skin cancers: a 7.5% chance by age 20 and 90% by age 50. However, these SCCs appear to develop as a result of chronic wounds.
- Albinism where individuals have reduced pigment formation also results in an increased risk of developing SCCs, however, the level of risk has not been established.
Immunosuppression
Those who are chronically immunosuppressed have an increased risk of SCC. Transplant recipients have a substantially increased risk of developing SCC which has been estimated at 65-250 times the general population. In Australia, 45% of transplant recipients develop an SCC within 10 years of receiving their transplant.
Chronic wounds and inflammation
Chronic wounds and chronic inflammation are predisposed to developing SCC, this includes chronic leg ulcers and lichen sclerosis. SCCs developing from a chronic wound, tend to have a worse prognosis.
Smoking
Overall, smoking increases the risk of developing an SCC, however, conflicting studies have estimated the risk at anywhere between 20% and 200%. The risk increases with heavier smokers.
Human Papilloma Virus
HPV is a strong carcinogen and is responsible for about 95% of all cervical cancers. It is likely that it also causes cutaneous SCC and an association has been found, however, a causal link has not been definitively proven.
Diet
While some studies have suggested an increased risk of SCC with higher fat intake, others have conflicted with this notion.
How does a squamous cell carcinoma develop (Pathogenesis)?
SCCs develop from a complex interaction of genes and environmental influences, in particular UV-induced DNA damage. The exact mechanism of SCC development has not been identified, however, several genetic mutations have been identified, mostly related to UV-mediated DNA damage of tumour suppressor genes. The main genes involved include p53 (66%), NOTCH1/2 (40%), FAT1 (30%) and CDKN2A (35%). P53 mutations are involved in 50% of all cancers.
Mutations in these genes within squamous cells of the epidermis allow uncontrolled replication and growth of affected cells resulting in the development of SCC.
How aggressive is a squamous cell carcinoma (Grade)?
Cellular differentiation describes the process by which a stem cell becomes a specialised cell of the body. Squamous cells of the epidermis have differentiated into their specialist cell type for the skin.
Grade describes how well differentiated and mature the SCC cells are. Less differentiated SCCs are more aggressive and higher risk.
- Well-differentiated SCCs are the most differentiated and least aggressive. Because they have retained functional attributes of squamous cells they still produce significant amounts of keratin or crust.
- Moderately differentiated SCCs sit in the middle of differentiation and aggression.
- Poorly differentiated SCCs have minimal attributes of squamous cells and produce minimal if any keratin (crust). They are classed as high-risk, aggressive cancers.
What are the symptoms of a squamous cell carcinoma (clinical features)?
SCCs can develop anywhere on the skin and usually in areas most exposed to the sun. Unusually, in those with darkly pigmented skin, SCCs are actually more common in non-sun-exposed areas.
SCCs usually present as flesh-coloured, scaly/crusty lumps that grow over a period of weeks to months. However, as per the grades above, a poorly differentiated squamous cell carcinoma may not have any crust.
SCCs can break down/ulcerate and ooze, they can be painful or painless.
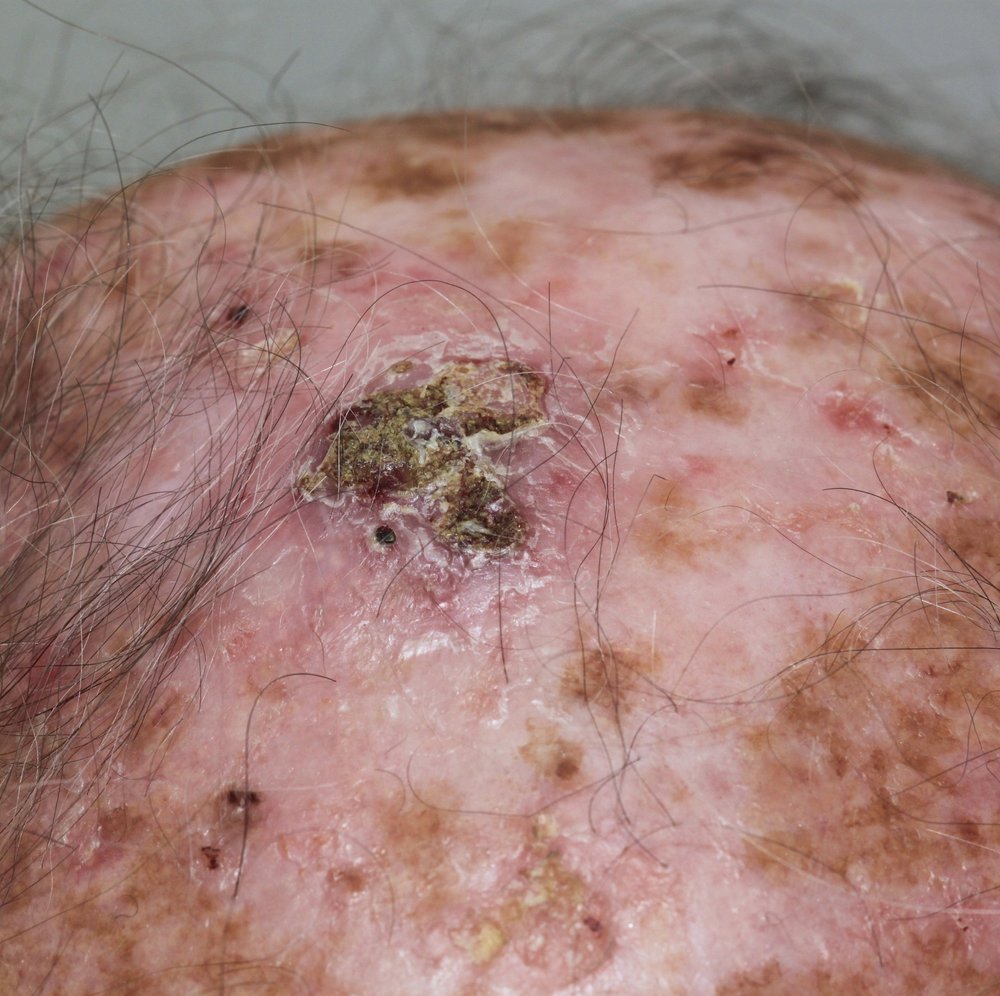
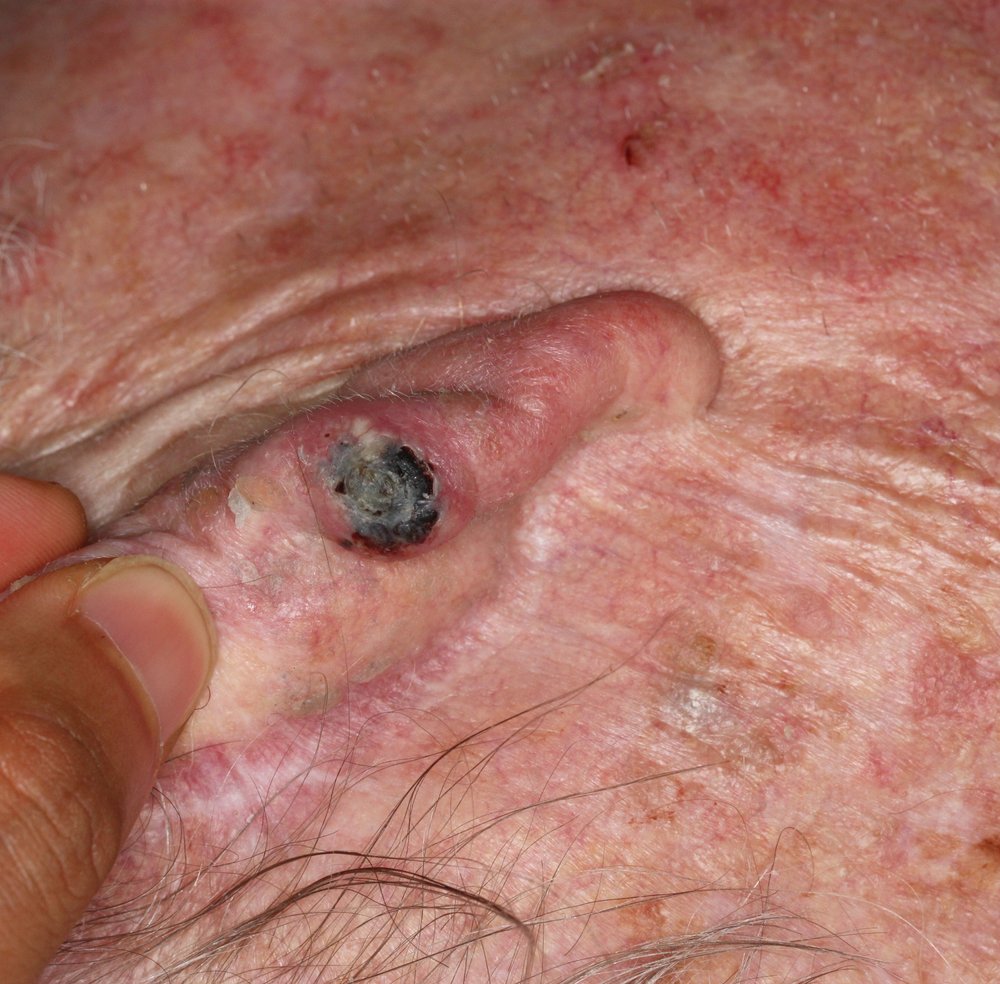
Squamous cell carcinoma variants
While most SCCs present as the typical type, there are some known variants of SCC that exhibit specific characteristics.
- Keratoacanthoma resemble SCCs however there is debate about whether they are a subtype of well differentiated SCCs or a separate condition. They tend to grow rapidly over weeks in a dome-shaped nodule with a central keratin core or plug. They can cause discomfort.
- Verrucous carcinoma resembles a cauliflower or wart-like lesion however it is rare.
- Marjolin’s ulcer is an SCC that develops from sites of chronic inflammation or wounds like leg ulcers. They can take several years to develop from the wound. They tend to have a poor prognosis.
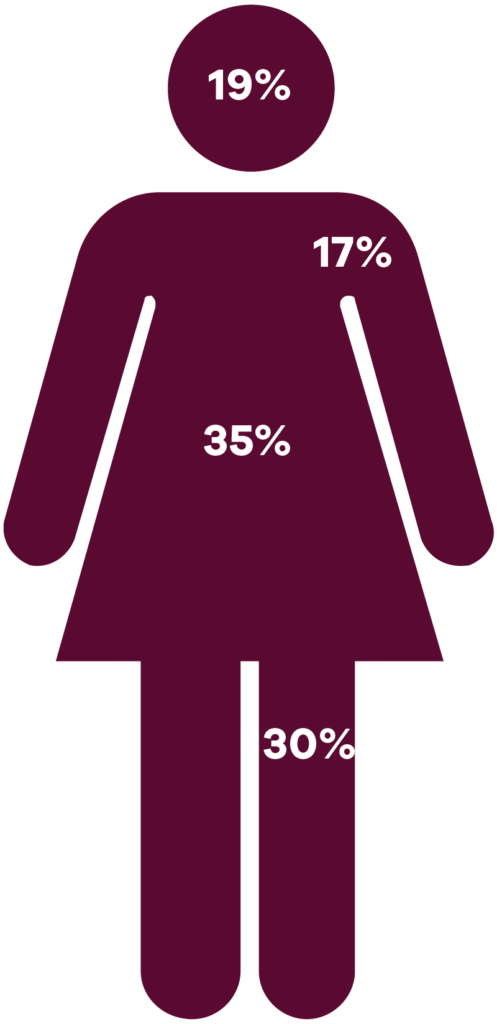
Where does a squamous cell carcinoma occur on the body?
Adjusted for surface area, the face, head and neck is the most common site for SCCs, followed by the trunk.
How is a squamous cell carcinoma diagnosed?
A lot of the time an experienced dermatologist will be able to diagnose SCCs clinically (without a biopsy). Nonetheless, it is sometimes still prudent to confirm the diagnosis with a biopsy.
There are many types of biopsies that can be performed. The key is to provide an adequate tissue sample that is representative of the lesion for the laboratory to make the diagnosis and provide adequate information to make treatment decisions, e.g. depth, how aggressive it is etc:
- Punch biopsy is the gold standard, however, will normally require stitches meaning that you will need to rest and look after the wound. You will need to return to the clinic a week later for stitches to be removed which is often inconvenient for people. There is also a risk of bleeding with this type of biopsy.
- Shave or curettage biopsies are an alternative option, provided the doctor takes care to obtain an adequate sample for the laboratory. The advantage of this biopsy is that it is superficial, requires no rest period and patients don’t need to return to the clinic for removal of sutures. However, they provide limited information on depth.
Once obtained, the sample is fixed in formalin, stained with haematoxylin and eosin (H&E) and prepared on slides. The pathologist will then examine these slides under a microscope and issue their report.
Treatment of a squamous cell carcinoma
Treatment planning is undertaken in conjunction with your doctor and will take into consideration a number of factors. These include the depth, size, location and stage of the squamous cell carcinoma as well as your personal preference. Your life situation may also come into consideration.
An SCC will normally require surgical excision or Mohs surgery to be treated adequately, however, in special circumstances, alternative treatments may be required.
Risk stratification
Prior to treatment, risk stratification will be undertaken to determine whether the SCC is low or high risk which will in turn guide treatment. A range of characteristics will help determine the risk level. The National Comprehensive Cancer Network (NCCN) risk stratification is:
- Low-risk: Lesions < 2 cm in diameter on trunk or extremities & well or moderately differentiated
- High-risk:
- Lesions > 2 cm in diameter on trunk or extremities
- Lesion of any size on head, neck, hands, feet, shin, anogenital region
- Recurrent SCC
- Acantholytic, adenosquamous or metaplastic subtypes
- Perineural or vascular invasion
- Very high-risk:
- Lesions > 4 cm in diameter
- Poorly differentiated, desmoplastic, > 6 mm deep or invasion into fat or nerve sheath
- Lymphatic or vascular invasion
Lip and ear lesions also have a higher risk of recurrence, as do people who are immunosuppressed. SCCs in those with epidermolysis bullosa (EB) are particularly aggressive and are the most common cause of death in those with EB.
Field cancerisation
Field cancerisation is when a large area of skin is carcinogenic and often presents on the lower legs. In this situation, new SCCs can be triggered very easily with minimal trauma (e.g., a scratch) or inflammation. Furthermore, even when it is repeatedly excised completely new SCCs appear within the healing wound which can cause significant concern.
This situation will need to be recognised and the area is often pre-treated with a field treatment to ‘clean up’ the skin prior to surgery.
Curettage and Cautery
Some people may not wish to undergo curative surgery to remove the cancer. For instance, they may already be debilitated with other medical problems and simply want minimal treatment to reduce the pain from an SCC. In this situation a curettage and cautery may be an appropriate option, however, it should not be considered a definite treatment and should be framed as a debulking, slowing down the SCC or simply a palliative treatment.
Curettage and cautery is a quick, minimally invasive surgical procedure that can be thought of as superficially ‘scraping’ the cancer. However, it is not possible to perform tissue analysis to confirm the complete removal of the SCC and cure rates are low.
It also generally results in a poor cosmetic outcome commonly resulting in broad, white and depressed scars. Nonetheless, it may be a preferred option for older patients who are unconcerned about cosmesis or recurrence in their expected lifetime.
Surgery
For most SCCs, surgical excision is recommended. The two main surgical techniques are a wide local excision and Mohs surgery. Surgery is normally performed under local anaesthetic and requires care (rest period) for a stitched wound.
A WLE is a reasonable option for low-risk SCCs on the trunk or limbs. We discuss WLEs in more detail in a separate page. The recurrence rate for WLE is approximately 5-15% however, this is dependent on:
- The SCC grade as more aggressive SCCs have higher recurrence rates.
- Tumour location and size.
- The surgeon’s incomplete excision rate. We have a page dedicated to why this is important here.
- A range of less significant risk factors.
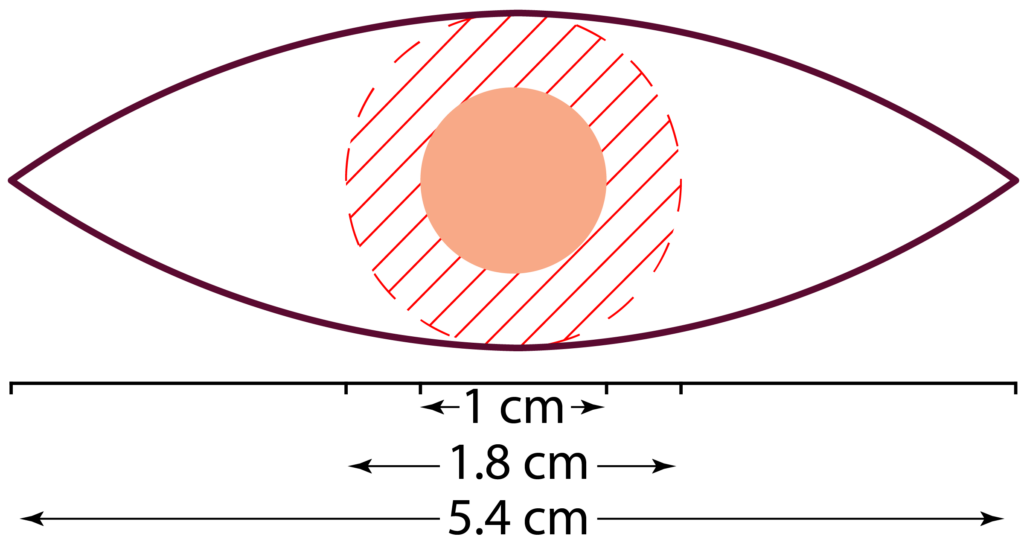
A wide local excision (WLE) is normally performed under local anaesthetic. The surgeon marks out the lesion based on their visual inspection. The NCCN recommend 4-6 mm margins and a depth of mid to deep subcutaneous fat. However, for high-risk SCCs, the margins of 6 to > 10 mm are recommended. The incision will be made in a 3:1 ellipse around this safety margin. So, an SCC with a 1 cm diameter will result in a scar line approximately 5.5 cm long (on cylindrical areas e.g., the arm, the ratio may be 5:1, meaning a scar line of about 9 cm).
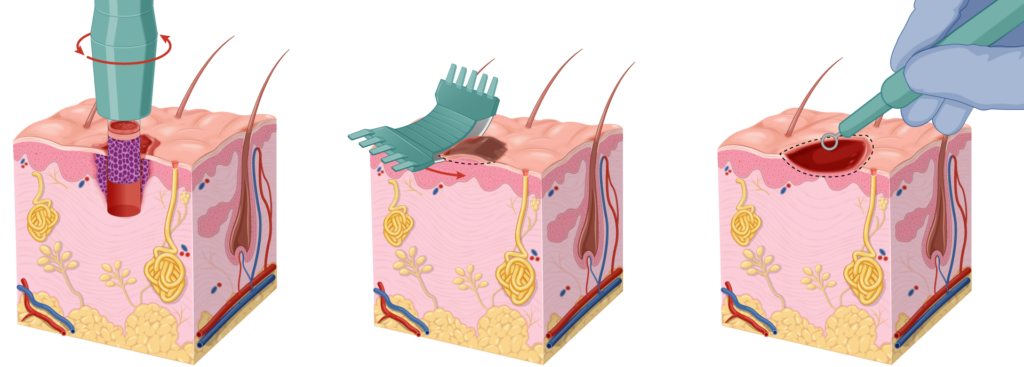
Mohs Surgery
Mohs micrographic surgery (MMS) is the gold standard treatment for SCCs of the head & neck, digits and genitals, especially lesions that are considered higher risk. Mohs can also be used on the trunk or limbs for difficult, high-risk or recurrent SCCs.
Mohs surgery has a better cure rate than standard surgery (WLE) – the recurrence rate is 1% for most lesions but is around 2-3% for high-risk lesions.
Mohs surgery has some key advantages:
- It keeps the surgical defect and wound as small as possible – this is particularly important in cosmetically sensitive areas. If you have an SCC on the tip of the nose, a bigger wound can have a significant cosmetic impact.
- Specimen (histological) analysis is undertaken while you wait. This means you don’t need to return at a later date for further surgery – it is all sorted out on the same day.
- Higher cure rate compared to standard surgery (WLE)
Radiotherapy
Radiotherapy is an alternative option to surgery for those who are unable to tolerate a surgical procedure. The radiation is delivered over a course of treatment, requiring multiple trips to the hospital. The cure rate for radiotherapy is about 93% for previously untreated SCCs.
Furthermore, radiotherapy tends to have worse cosmetic outcomes compared to surgery as radiotherapy fields tend to degrade over time.
Radiotherapy may be used as an adjunct to surgery where the tumour was unable to be completely removed or for high-risk lesions to reduce the risk of future recurrence. It is particularly used in tumours with significant perineural invasion – when the cancer is starting to track around nerve fibres.
Recurrent tumours
It is important not to keep treating the SCC with the same treatment modality if the tumour recurs and alternative options are available. For example, if the cancer recurs after treatment with cryotherapy or Efudix, the doctor needs to consider whether this was an appropriate treatment for this tumour in the first place and whether the SCC was invading deeper than what was assumed. In the case of recurrence after non-invasive treatments, strong consideration should be given to further treatment with surgical approaches.
Advanced stage SCCs
If it is no longer localised to the skin and subcutaneous tissue, treatment will be more complex and is often undertaken in the context of a multidisciplinary team (MDT). Further investigations may be required (eg. CT/MRI scans) to determine what structures (eg. nerves, muscle, bone) are involved which will guide further treatment.
Surgery is still often used, however, advanced SCCs may require a general anaesthetic to remove with complex surgery. Surgery may be followed by radiotherapy and systemic treatments.
Prognosis & complications of a squamous cell carcinoma
If left, SCCs will start to break down into a chronic wound causing recurrent bleeding or ooze requiring regular dressings. They can cause problems by invading adjacent structures (bones, muscles, etc) or by travelling down nerves (perineural invasion). About 5% of SCCs spread (metastasise) to other areas of the body. SCCs can recur after treatment, often over a larger area than the original.
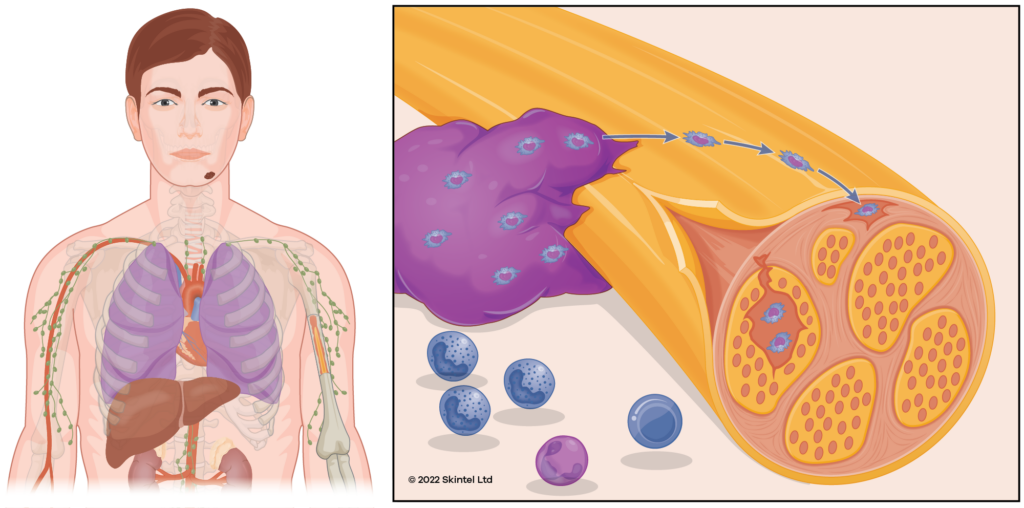
It is common for people to develop new SCCs: about 30% of people will develop another one within five years of an SCC diagnosis. However, the risk of any subsequent skin cancer is about 50%. As a result, we recommend regular skin checks at least every twelve months.
Interestingly, people who have developed an SCC have an increased risk of developing other cancers such as lung, bowel, and breast cancer. This risk is about double the general population. However, screening for these malignancies is not recommended in those who are otherwise well.
Prevention of developing a squamous cell carcinoma
The main approach to prevention involves sun protection. Some studies have suggested that rigorous sun protection during childhood can reduce the development of SCCs by 78% but this level of reduction has not yet been conclusively proven. Daily sunscreen use has been shown to reduce the risk of developing one by 38%. Obviously, daily sunscreen use requires a significant commitment from individuals – even dermatologists are not perfect with their sun protection.
General sun protection advice includes:
- Avoid sunburn and intentional tanning.
- Sun protective clothing – hat, sunglasses.
- Seek shade where available.
- Extra care needs to be taken in highly reflective environments e.g., water, snow, sand.
- Vitamin D should be obtained through food and supplements.
- Broad-spectrum, water-resistant sunscreen with a minimum of SPF50.
We have separate pages dedicated to sun protection and sunscreen. These pages cover controversial topics such as the impact of vitamin D, high-SPF sunscreens and the absorption of sunscreen ingredients.
Some studies have suggested that the use of nicotinamide (vitamin B3) may help reduce the risk of developing SCCs by 30%, however, subsequent statistical analysis has called into question the validity of this study. Further studies are required to establish the efficacy of nicotinamide.
Other Resources
References
- Dubas LE, Ingraffea A (2013). Nonmelanoma skin cancer. Facial Plastic Surgery Clinics of North America. 21 (1): 43-53. doi: 10.1016/j.fsc.2012.10.003
- Olsen CM, Pandeya N, Green AC, Ragaini BS, Venn AJ, Whiteman DC. Keratinocyte cancer incidence in Australia: a review of population-based incidence trends and estimates of lifetime risk. Public Health Res Pract. 2022;32(1):e3212203. doi: 10.17061/phrp3212203
- Gandhi SA, Kampp J (November 2015). “Skin Cancer Epidemiology, Detection, and Management.” The Medical Clinics of North America. 99 (6): 1323-35. doi: 10.1016/j.mcna.2015.06.002
- Stang, A., Khil, L., Kajüter, H., Pandeya, N., Schmults, C. D., Ruiz, E. S., & Green, A. C. (2019). Incidence and mortality for cutaneous squamous cell carcinoma: comparison across three continents. Journal of the European Academy of Dermatology and Venereology, 33(S8), 6-10. doi: 10.1111/jdv.15967
- Abroms L, Jorgensen CM, Southwell BG, et al. Gender differences in young adults’ beliefs about sunscreen use. Health Educ Behav. 2003 Feb;30(1):29-43. doi: 10.1177/1090198102239257
- McCarthy, W. H. (2004). The Australian experience in sun protection and screening for melanoma. Journal of Surgical Oncology, 86(4), 236-245. doi: 10.1002/jso.20086
- Zanetti R, Rosso S, Martinez C, et al. Comparison of risk patterns in carcinoma and melanoma of the skin in men: a multi-centre case-case-control study. Br J Cancer 2006; 94:743. doi: 10.1038/sj.bjc.6602982
- George EA, Baranwal N, Kang JH, et al. Photosensitizing Medications and Skin Cancer: A Comprehensive Review. Cancers (Basel) 2021; 13. doi: 10.3390/cancers13102344
- Kishikawa M, Koyama K, Iseki M, et al. Histologic characteristics of skin cancer in Hiroshima and Nagasaki: background incidence and radiation effects. Int J Cancer 2005; 117:363. doi: 10.1002/ijc.21156
- Gloster HM Jr, & Neal K. Skin cancer in skin of color. J Am Acad Dermatol 2006; 55:741. doi: 10.1016/j.jaad.2005.08.063
- Hussain SK, Sundquist J, & Hemminki K. The effect of having an affected parent or sibling on invasive and in situ skin cancer risk in Sweden. J Invest Dermatol 2009; 129:2142. doi: 10.1038/jid.2009.31
- Wehner MR, Linos E, Parvataneni R, et al. Timing of subsequent new tumors in patients who present with basal cell carcinoma or cutaneous squamous cell carcinoma. JAMA Dermatol 2015; 151:382. doi: 10.1001/jamadermatol.2014.3307
- Kraemer KH, Tamura D, Khan SG, & Digiovanna JJ. Burning issues in the diagnosis of xeroderma pigmentosum. Br J Dermatol 2013; 169:1176. doi: 10.1111/bjd.12707
- Fine JD, Johnson LB, Weiner M, et al. Epidermolysis bullosa and the risk of life-threatening cancers: the National EB Registry experience, 1986-2006. J Am Acad Dermatol 2009; 60:203. doi: 10.1016/j.jaad.2008.09.035
- Jensen P, Hansen S, Møller B, et al. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol 1999; 40:177. doi: 10.1016/s0190-9622(99)70185-4
- Berg D, Otley CC. Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. J Am Acad Dermatol 2002; 47:1. doi: 10.1067/mjd.2002.125579
- Tobin C, Sanger JR. Marjolin’s Ulcers: A Case Series and Literature Review. Wounds 2014; 26:248. pmid: 25860780
- Youl, P. H., Janda, M., Aitken, J. F., Del Mar, C. B., Whiteman, D. C., & Baade, P. D. (2011). Body-site distribution of skin cancer, pre-malignant and common benign pigmented lesions excised in general practice. British Journal of Dermatology, 165(1), 35-43. doi: 10.1111/j.1365-2133.2011.10337.x
- Stratigos AJ, Garbe C, Dessinioti C, et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 2. Treatment. Eur J Cancer 2020; 128:83. doi: 10.1016/j.ejca.2020.01.008
- Krausz AE, Ji-Xu A, Smile T, et al. A Systematic Review of Primary, Adjuvant, and Salvage Radiation Therapy for Cutaneous Squamous Cell Carcinoma. Dermatol Surg 2021; 47:587. doi: 10.1097/DSS.0000000000002965
- Lovett RD, Perez CA, Shapiro SJ, Garcia DM. External irradiation of epithelial skin cancer. Int J Radiat Oncol Biol Phys 1990; 19:235. doi: 10.1016/0360-3016(90)90529-s
- Joseph MG, Zulueta WP, Kennedy PJ. Squamous cell carcinoma of the skin of the trunk and limbs: the incidence of metastases and their outcome. Aust N Z J Surg 1992; 62:697. doi: 10.1111/j.1445-2197.1992.tb07065.x
- Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol 2000; 136:1524. doi: 10.1001/archderm.136.12.1524
- Chen J, Ruczinski I, Jorgensen TJ, et al. Nonmelanoma skin cancer and risk for subsequent malignancy. J Natl Cancer Inst 2008; 100:1215. doi: 10.1093/jnci/djn260
- Stern RS, Weinstein MC, Baker SG. Risk reduction for nonmelanoma skin cancer with childhood sunscreen use. Arch Dermatol 1986; 122:537. doi: 10.1001/archderm.1986.01660170067022
- Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol 2011; 29:257. doi: 10.1200/JCO.2010.28.7078
- Chen AC, Martin AJ, Choy B, et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N Engl J Med 2015; 373:1618. doi: 10.1056/NEJMoa1506197
Related Posts
-
Does Nicotinamide Help with Skin Cancer?
An in-depth review of the evidence for and against supplementing with nicotinamide in an effort to reduce the risk of developing skin cancer.Read more
-
What a Mohs Surgeon sees under the Microscope
Experience matters when it comes to picking out a tumour under the microscope. The Skintel Mohs specialists have viewed more than 10,000 slides and counting! […]Read more
-
Skin Cancer: A Basic Guide
Skin cancer is by far the most common type of cancer. They are most common in areas with lots of sun exposure such as the face, neck, arms, and head.Read more

